The Next-Generation Technology Expediting Mouse Model Creation
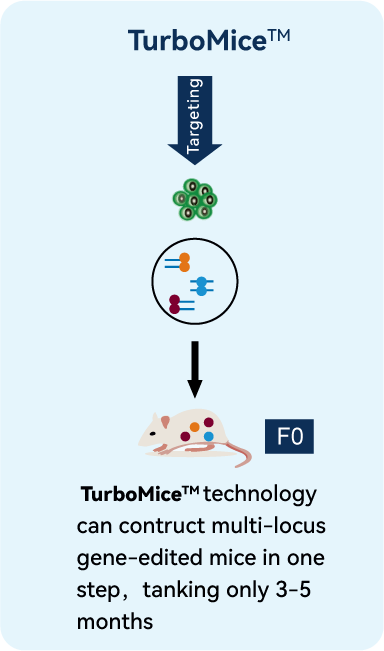
TurboMice™ Technology
Developed by MingCeler through a series of optimizations of tetraploid complementation.
By combining Precise Gene Editing technology and optimized mouse Embryonic Stem Cell Preparation technology, we can now edit almost any target gene locus.
TurboMice™ Technology
Our highly efficient Tetraploid Complementation technology has allowed us to greatly increase the birth rate of mice from 1%-5% to 30%-60% with gene-edited stem cells, nearly matching the efficiency of normal embryo transfer.
MingCeler is the first company in the world to successfully accomplish the transformation of Tetraploid Complementation technology from laboratory to industrial application.
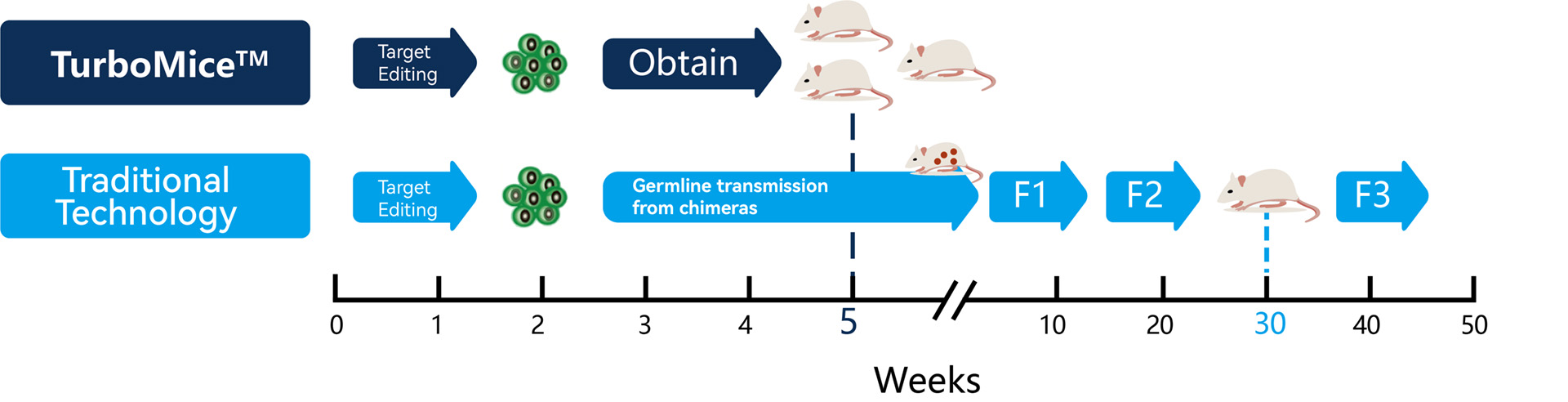
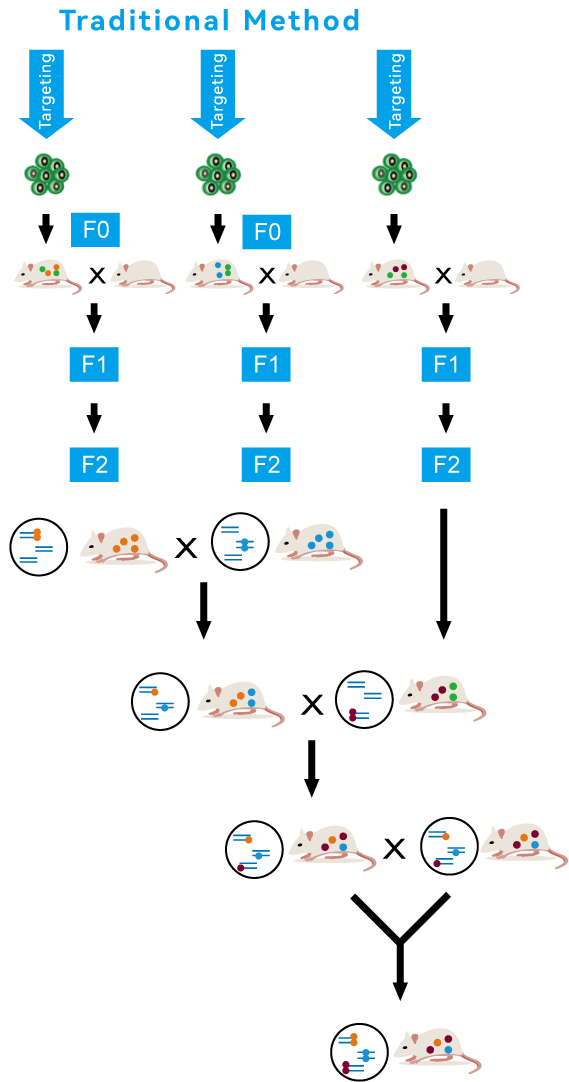
Traditional Technology
Traditional animal modeling technology, such as pronuclear microinjection and ES targeting, involves breeding at least 2 to 3 generations to obtain mice of a homozygous genotype, which typically takes up 6-8 months.
This lengthy process significantly hampers the progress and efficiency of new drug development and disease research.
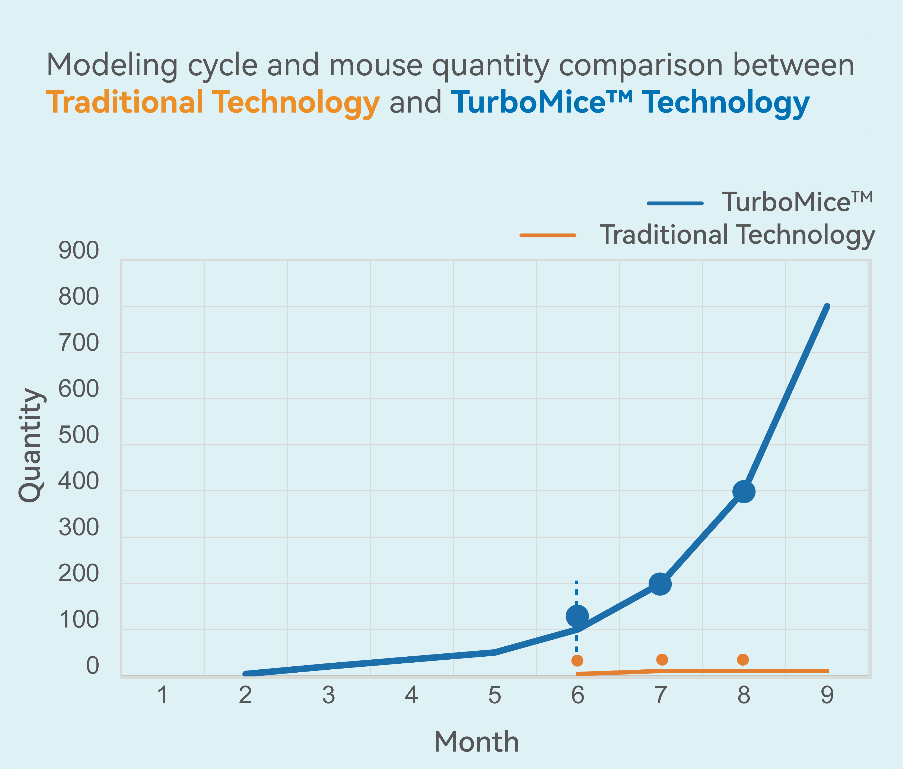

Quick Batch
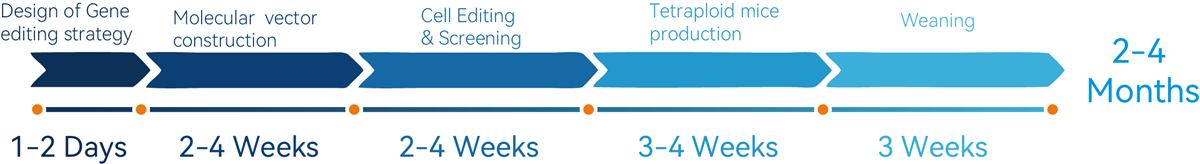
1. up to 20 homozygous gene-edited mice within 2-4 months.
2. ≈ 50 homozygous mice within the following 2 months.
3. 400+ homozygous mice within 8-12 months.
Case Report in 2020
TurboMice™ technology was leveraged to rapidly produce 500 homozygous humanized ACE2 mice within just 8 months for COVID-19 drug and vaccine studies.
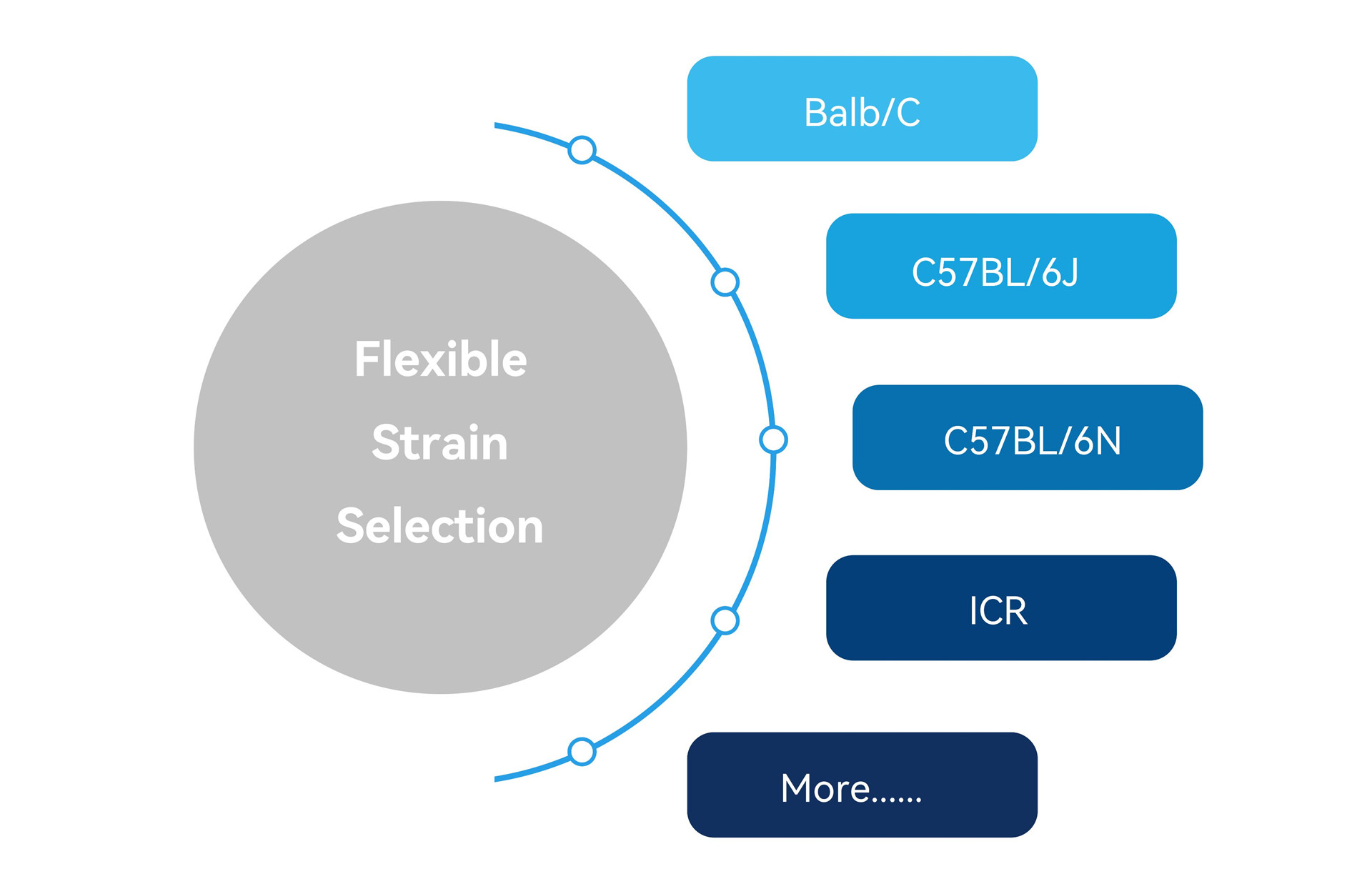
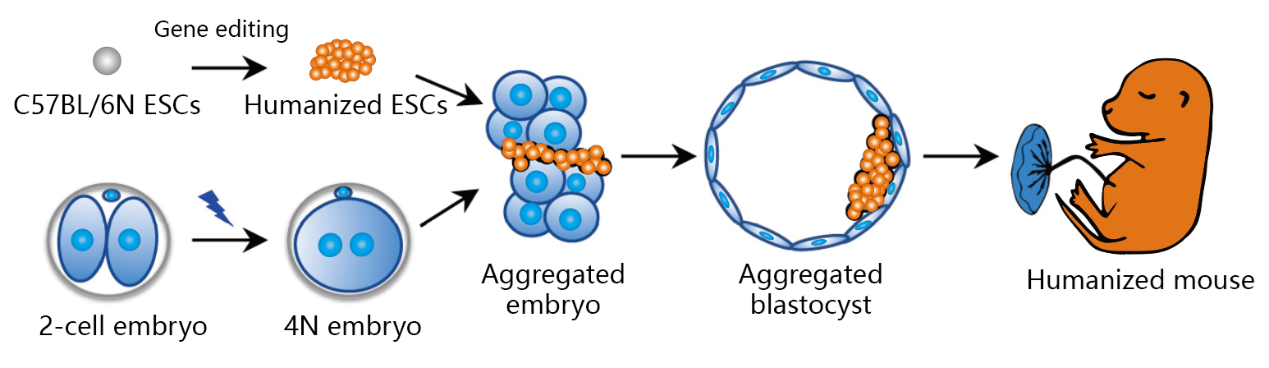
Flexible Strain Selection
Traditional techniques have significant limitations in mouse strain selection, whereas TurboMice™ technology offers more flexibility with a variety of inbred and outbred strains to choose from (including Balb /c, ICR, C57BL/6, etc.).
● F0 generation mice constructed using TurboMice™ technology are the target mice with single-cell origin.
● Therefore, the genetic material of the F0 mice is identical, reducing the experimental errors caused by genetic material variabilities.
● Ergo, the experimental data for drug efficacy evaluation and other experiments are more consistent and reliable.

Maintains Good Genetic Integrity
In Situ Precision Gene Editing
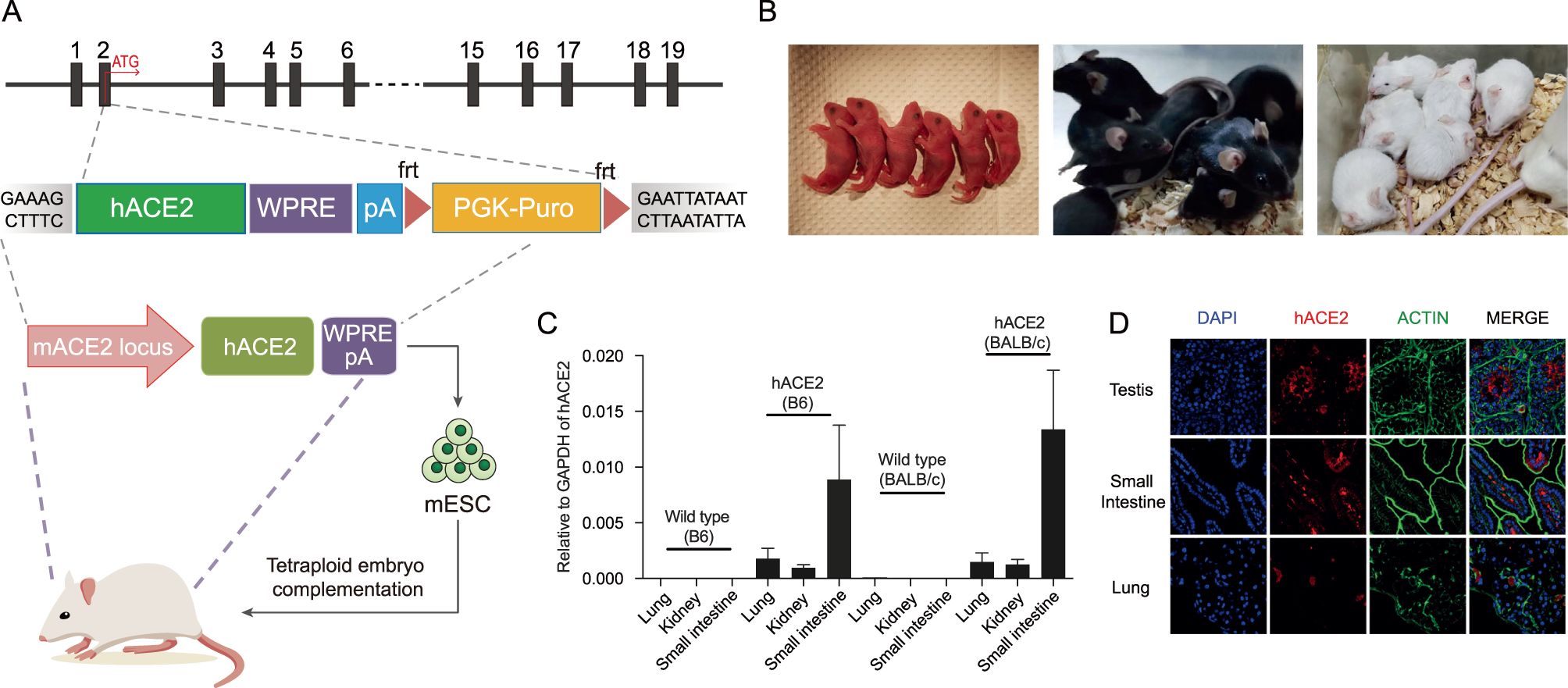
● In situ precise gene editing with precise gene expression levels and accurate tissue specificity.
● Humanized ACE2 mice developed through traditional techniques are generated by the exogenous introduction of the K18-ACE2 promoter, which is unable to achieve precise editing due to random insertion, resulting in a lack of tissue specificity of ACE2 expression in the constructed humanized ACE2 mouse model.
● MingCeler's humanized ACE2 mice exhibit specific expression in different organs (graphics C and D above), and effectively simulate clinical characteristics following SARS-CoV-2 infection.

Unique EnhancerPlus Platform
● MingCeler's proprietary EnhancerPlus platform can help our customers improve the expression levels of humanized genes.
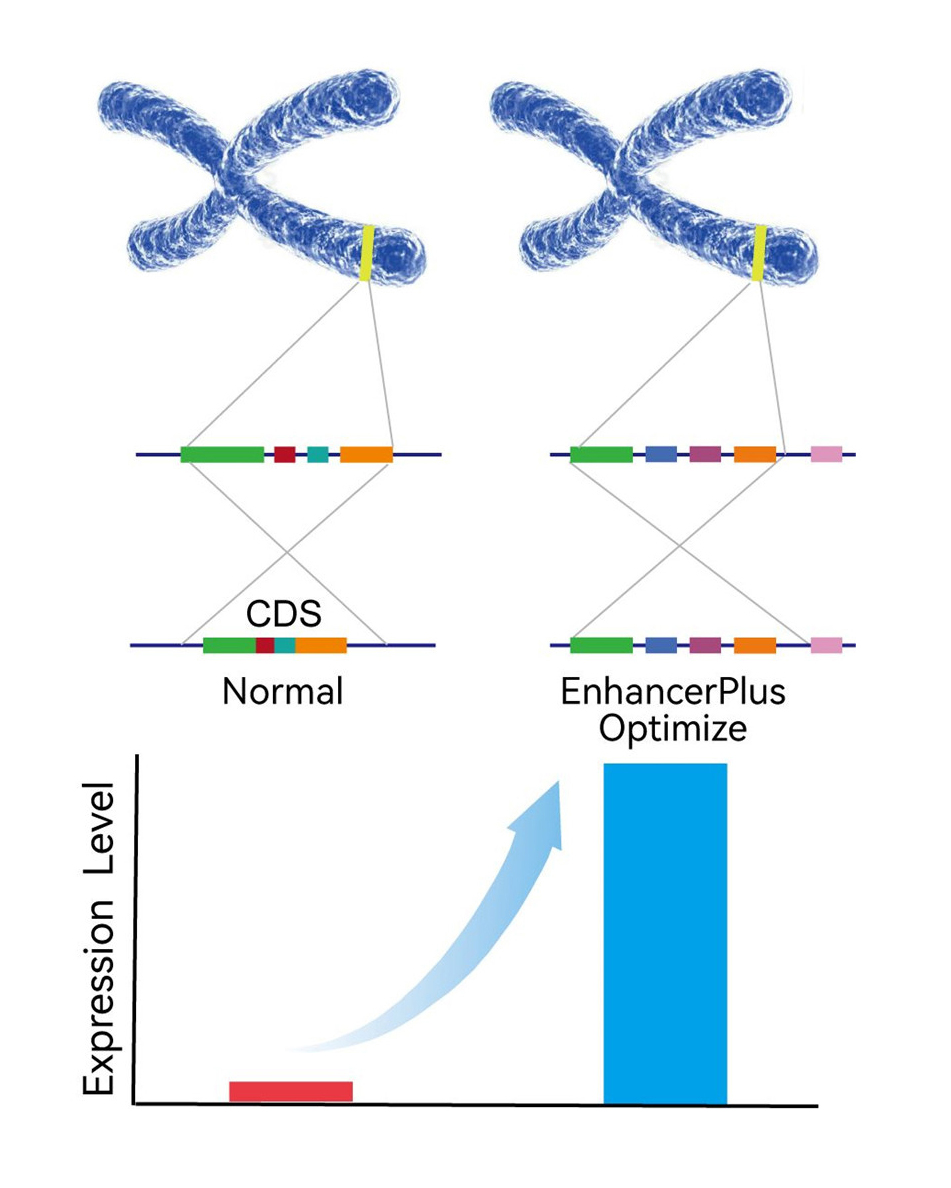
● Resultant of years of data accumulation in epigenetics and cell fate determination research, MingCeler's EnhancerPlus platform can accurately predict the genomic positions of Enhancers, making the design of gene editing strategies evidence-based.
● As a result, the target genes can better resemble the expression patterns and levels of endogenous genes.
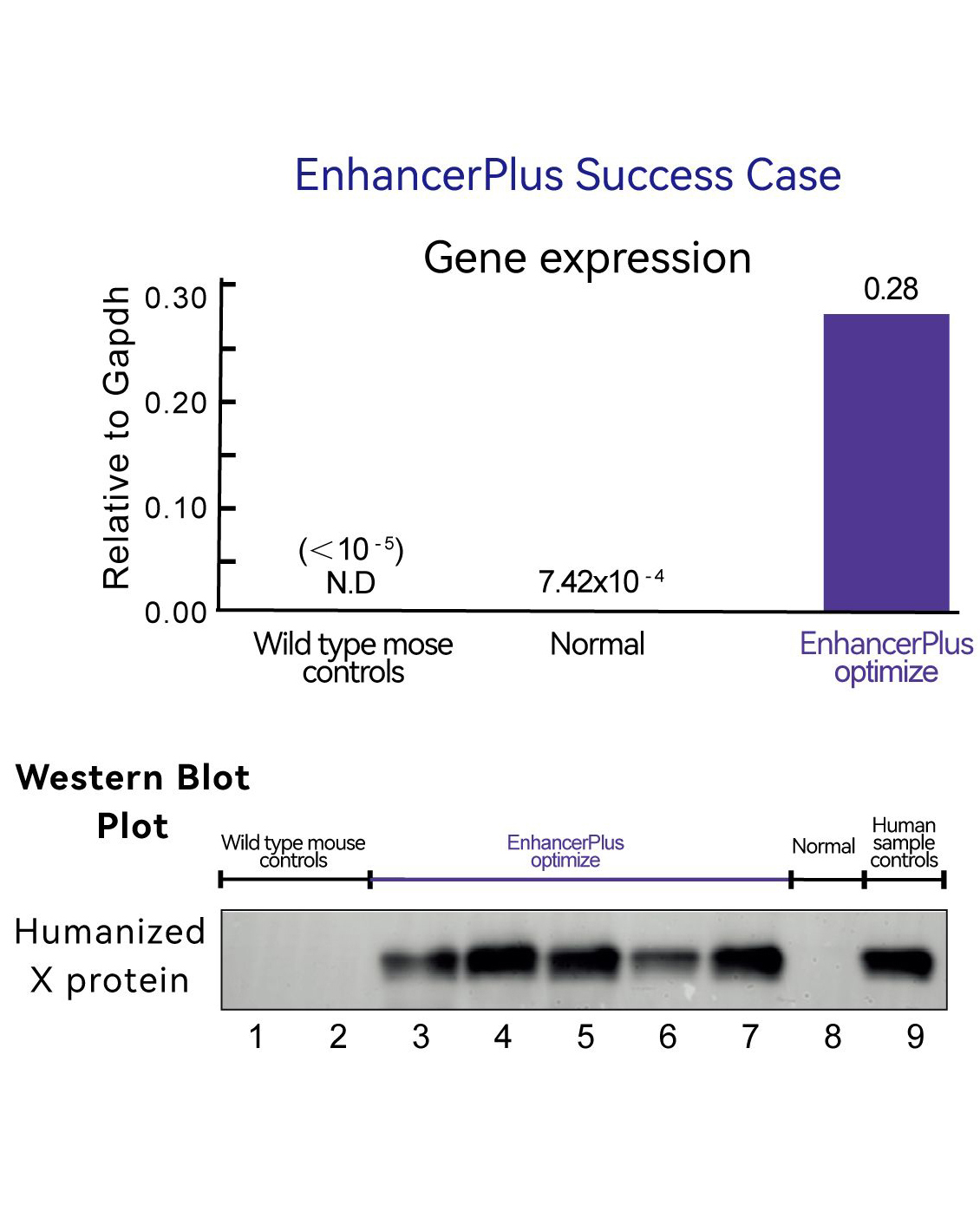
Case report: Humanization of X gene.
● Leveraging the EnhancerPlus platform, the strategy was optimized and the expression level saw an increase of three orders of magnitude (see above graphic), reaching the requirements for drug development as determined by the customer at the protein level.
● After completing the world's first mass-production of ACE2 humanized mice in 2020, MingCeler has upgraded the ACE2 humanized mouse model through four iterations via EnhancerPlus optimization, with the expression level of humanized ACE2 eventually reaching near the endogenous expression of mouse ACE2.
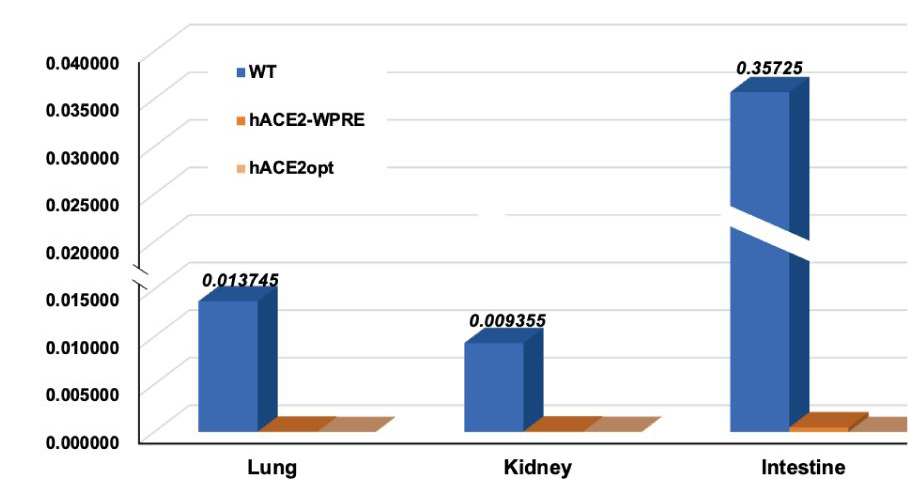
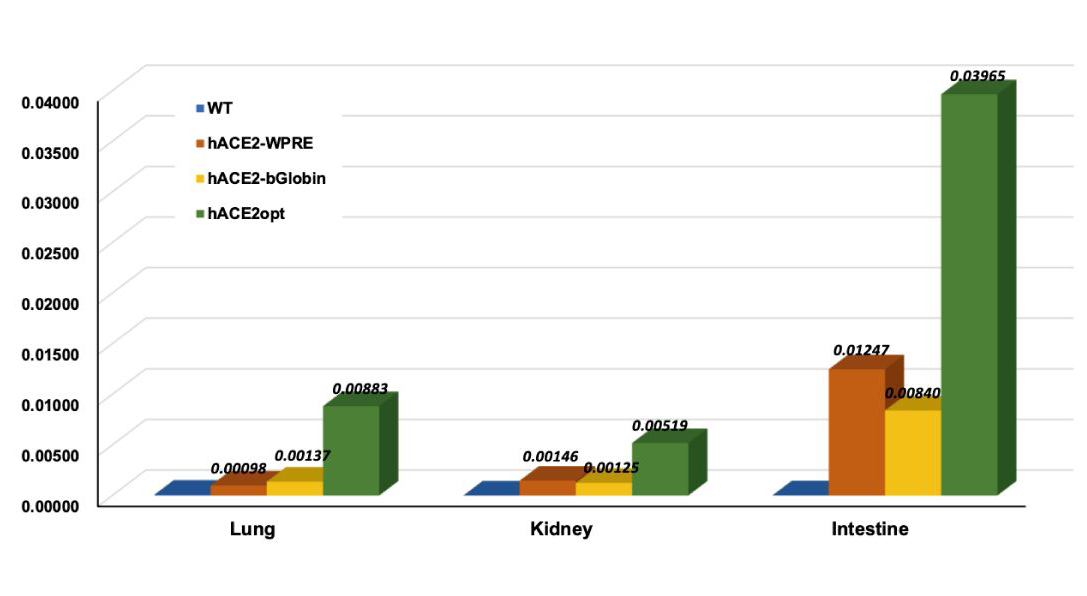
Expression levels of humanized ACE2 in different organs of the humanized mouse models

Multi-Locus Gene Editing
● TurboMice™ technology is the best choice on the market for generating multi-locus combinatorial gene-edited mouse models, giving the advantages of high accuracy in inserting location, long fragment insertion, and multiple genetically modified loci, with no allelic segregation issues.
● TurboMice™ technology allows for the simultaneous editing of up to 3 genes to produce homozygous multi-locus gene-edited mouse models directly from gene-edited embryonic stem cells, without the need for the time-consuming breeding/screening processes, providing a variety of high-value complex models required for innovative drug research.
● MingCeler has successfully developed various multi-loci gene-edited mouse models in a short time, based on ACE2 humanized mice.

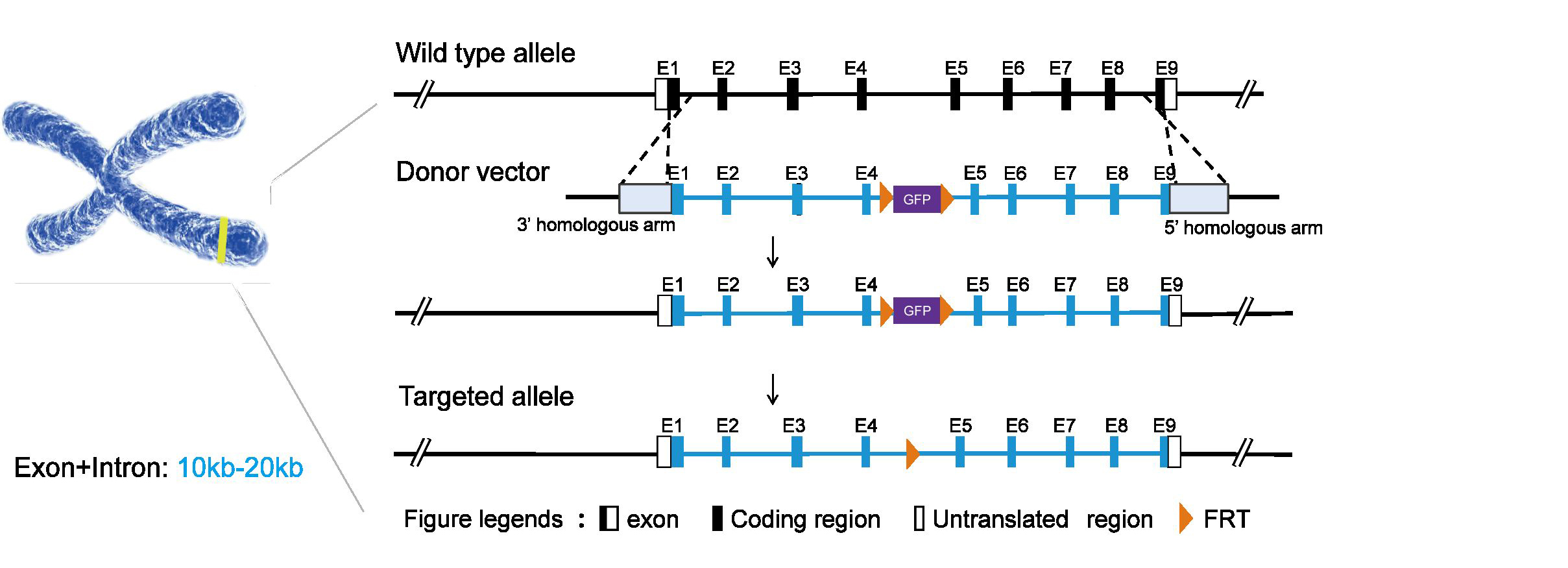
Long Fragment Gene Editing
TurboMice™ technology enables precise gene editing of long fragments of over 20kb, facilitating the rapid production of complex models such as humanization, conditional knock-out (CKO), and large fragment knock-in (KI).

Publications
[1] Wang G, Yang ML, Duan ZL, Liu FL, Jin L, Long CB, Zhang M, Tang XP, Xu L, Li YC, Kamau PM, Yang L, Liu HQ, Xu JW, Chen JK, Zheng YT, Peng XZ, Lai R. Dalbavancin binds ACE2 to block its interaction with SARS-CoV-2 spike protein and is effective in inhibiting SARS-CoV-2 infection in animal models. Cell Res. 2021 Jan;31(1):17-24. doi: 10.1038/s41422-020-00450-0. ( IF: 20.507 )
[2] Liu FL, Wu K, Sun J, Duan Z, Quan X, Kuang J, Chu S, Pang W, Gao H, Xu L, Li YC, Zhang HL, Wang XH, Luo RH, Feng XL, Schöler HR, Chen X, Pei D, Wu G, Zheng YT, Chen J. Rapid generation of ACE2 humanized inbred mouse model for COVID-19 with tetraploid complementation. Natl Sci Rev. 2020 Nov 24;8(2):nwaa285. doi: 10.1093/nsr/nwaa285. ( IF: 16.693 )
Service Flow

All you need to do is provide us your gene modification requirement for your mouse model. Based on your specifications, we will put together a customized preliminary plan, and following further discussion and mutual agreement, we will sign a technical service contract. After project commencement we will provide timely updates on progress.